
This is possible because carbon is capable of forming an enormous variety of stable chemical compounds, which is why it is considered by many to be the king of chemical elements.Ĭarbon is a chemical element, represented by the symbol C. The word "carbon" comes from the Latin "carbo" which designated coal and from the Roman "adamas" which means "hard steel". 1999‑2023 - All Rights Reserved.Carbon is one of the most important elements of the periodic table for living beings since it forms the basis of life as we know it. Īlso see: The Orbitron: A gallery of atomic orbitals and a few molecular orbitalsĬopyright © Israel Science and Technology Directory.
#Carbon electron configuration standard form full
The full story of the electron configurations of the transition elements. Thus, each proton and neutron has a mass of about 1 amu. This isotope of carbon has 6 protons and 6 neutrons. Atomic mass is measured in Atomic Mass Units (amu) which are scaled relative to carbon, 12C, that is taken as a standard element with an atomic mass of 12. Each element is uniquely defined by its atomic number.Ītomic mass: The mass of an atom is primarily determined by the number of protons and neutrons in its nucleus. Atomic number: The number of protons in an atom.
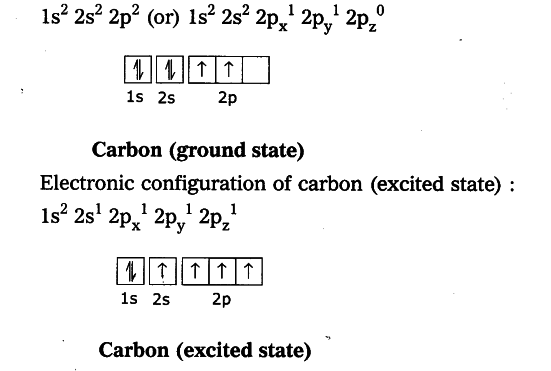
The table below shows the full forms of the electron configurations of noble gases. For an explanation of these aspects, see the reference by Schwarz listed below. Thus, the electron configuration of Sc is 3d 1 4s 2. Starting with Scandium (Sc, atomic #21), the 3d orbital has a lower energy than the 4s. Energy levels and sublevels Principal energy level Therefore, orbital 4s is filled with electrons prior to orbital 3d. The reason for this is that the energy level of orbital 4s is slightly lower than that of orbital 3d. Note that in the electron configuration of both K and Ca, the 4s orbital is filled before the 3d orbital. The one additional electron configuration completes the picture for 19 electrons of Potassium.
#Carbon electron configuration standard form plus
The abbreviated form - 4s 1 - means the electron configuration of Argon (Ar), plus one electron in the 4s orbital. The full electron configuration of Potassium (K) is 1s 22s 22p 63s 23p 64s 1. Thus, the configuration shown for Potassium is 4s 1 (see Table below). Thus, substituting the config of He gives the full config for Neon: 1s 22s 22p 6įor example, for Potassium (K) (atomic #19), the preceding noble gas is Argon (Ar) (atomic #18).
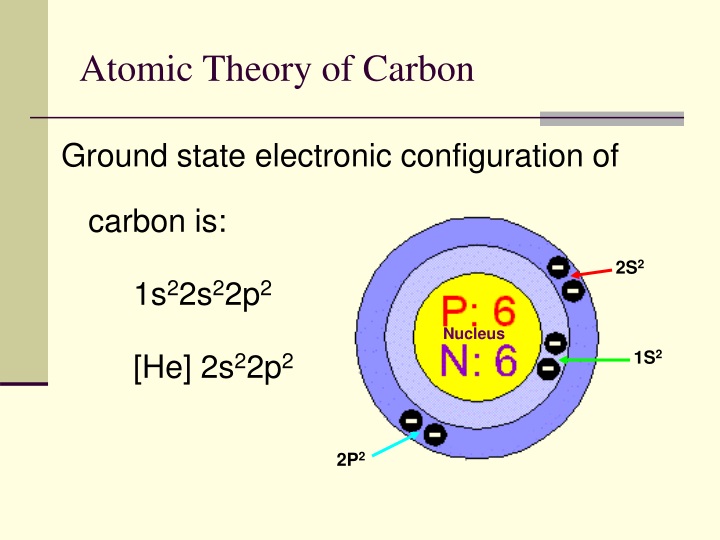

For example, the abbreviated configuration for Neon is 2s 2 2p 6. In the periodic table beyond Helium (He), each element's electron configuration is shown in an abbreviated form that starts with the symbol of the noble gas that precedes it. The superscript shows that there is one electron in the 1s orbital. The simplest configuration is for Hydrogen: 1s 1. An atom's electron configuration describes the distribution of its electrons in the atomic orbitals ordered by the orbitals' energy levels.


 0 kommentar(er)
0 kommentar(er)
